exploring the secretome for new metabolic hormones
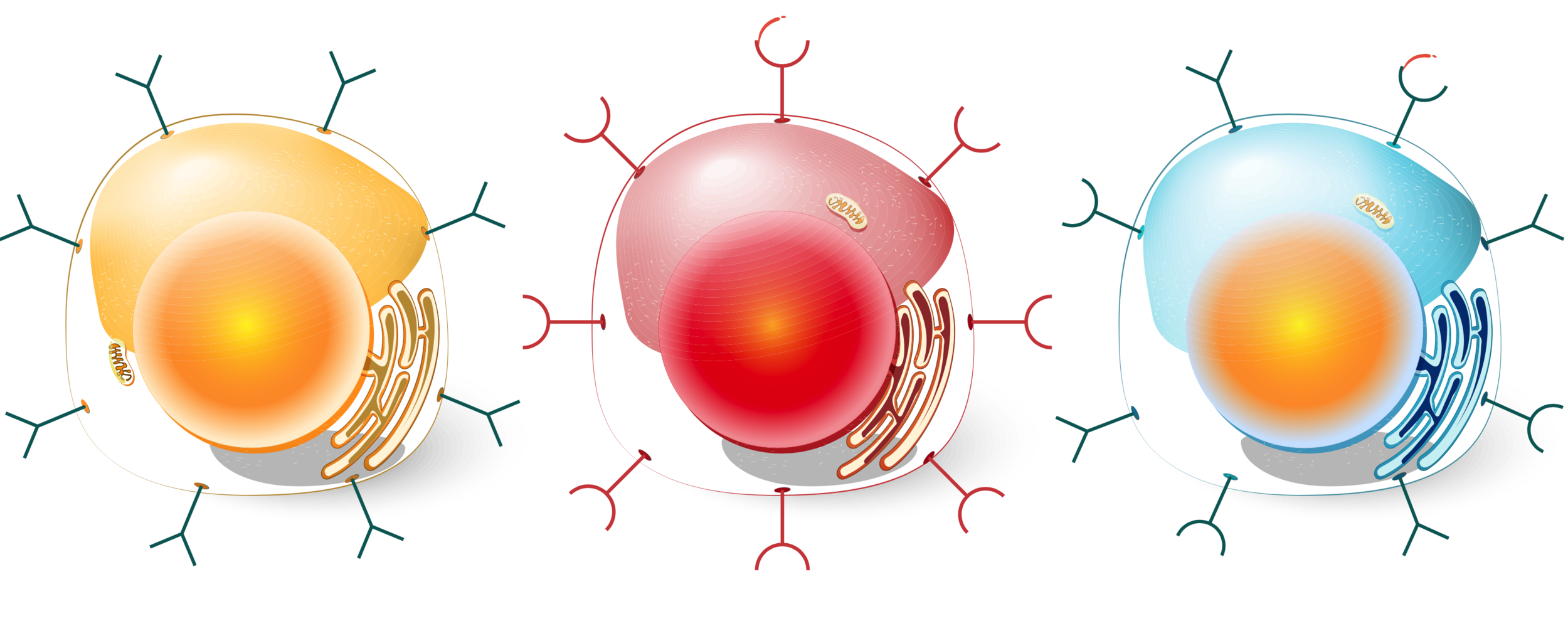
How do tissues communicate with each other, and how is this communication altered in disease? In the past decade, our understanding of growth factors (tissue-residing or blood-borne ) has vastly evolved, leading to the recognition of novel molecules with signaling properties from organs that were not previously considered to be part of the classical hormonal system (Zhao et. al. Frontiers in Physiology, 2020).
We use genomics, proteomics and cellular physiology to identify and study these new metabolic targets and their pathways. Our goals are to better understand complex physiological systems such as metabolism, cancer and aging.
identification of ligand-receptor pairs that regulate Metabolism
How do cells know which nutrients to take up and when? Our lab has discovered several adipose-derived secreted factors that control glucose or energy regulation (Jiang, Zhao, Voilquin et. al., Cell Metab, 2021)(Svensson et. al., Cell Metab, 2016). We are currently exploring these pathways by using proteomic approaches and secretome analyses to understand how peripheral tissues such as adipose, muscle, liver and small intestine communicate with each other.
Proteomics and Single-cell analysis to identify new metabolic drivers
Heterogeneity is a well-documented phenomenon resulting in cellular diversity and cell specialization in an organism. This heterogeneity is also contributing to differences in cellular signals and responses. We have developed protocols to isolate and study heterogeneous cell populations from adipose tissue and livers from mice with established metabolic diseases (Jung et al, STAR protocols 2020). We are using proteomics, single-cell approaches combined with CRISPR gene editing to resolve the cellular landscape during disease progression and to provide a resource for the development of novel therapeutic strategies.